
Working in Healthcare means risking exposure to biological agents throughout the day. While doctors, nurses or surgeons might be directly exposed to infection, other employees such as porters and cleaners might be exposed to indirect sources of infection. These risks exist in human healthcare (such as hospitals, clinics, and education establishments) as well as in animal healthcare (such as veterinary clinics, private practices, farms, and vet schools).
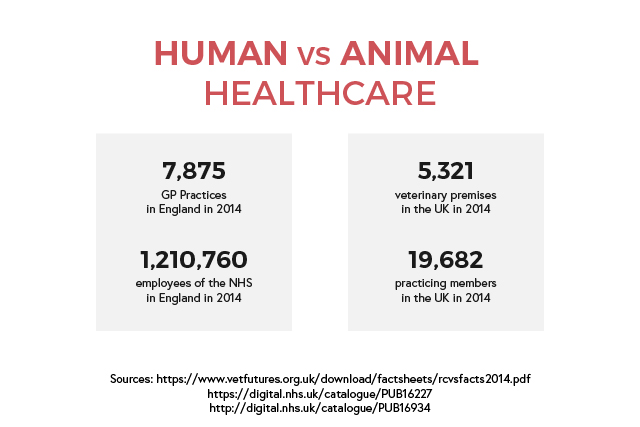
Human healthcare is a major industry in the UK, with employers in both public and private sectors. The NHS is the largest employer, with about a million employees in over 500 NHS trusts as well as primary care services. This includes general practitioners and dentists. The risk of infection in hospital employees is estimated to be low but infections are likely to be under-reported. However, a surveillance report shows that care assistants and attendants have the highest number of cases of work-related infections.
Animal healthcare is split between companion animals and food-producing animals. There are around fourteen thousand registered veterinary surgeons in the UK. The majority works in private practices while the government, universities, industry, and charities employ the rest. There is little to no information regarding the risk of infection, however an Austrian report shows that vets consider infections as ‘part of the job’ and go unreported.
This blog post aims to highlight the regulation requirements and the guidance documents to help comply with them, and focuses on the importance of CoSHH regulations. Because the subject is extensive, we will cover the steps in managing these risks in the next blog post!
How does CoSHH define biological agents and what work environments does it cover?
CoSHH defines a biological agent as ‘a micro-organism, cell culture, or human endoparasite, whether or not genetically modified, which may cause infection, allergy, toxicity or otherwise create a hazard to human health.’ Examples of micro-organisms that are biological agents are bacteria, viruses, fungi, microscopic endoparasites as well as larger endoparasites. The regulation classifies these biological agents based on whether they are pathogenic, a hazard for employees, are transmissible, and whether there is effective treatment for them.
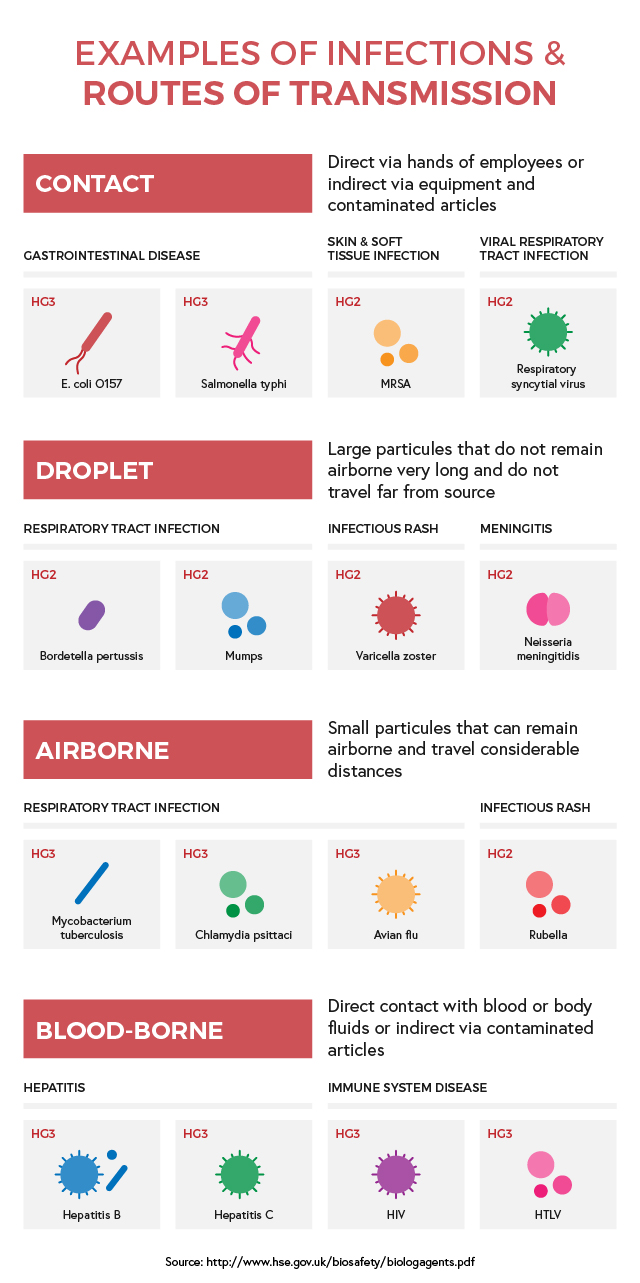
CoSHH then organises them into four Hazard Groups (HG):
- These biological agents are unlikely to cause human disease (Hazard Group 1)
- Bio-agents that can cause human disease and may be a hazard for employees, they are unlikely to be transmissible and there is an effective treatment for them (Hazard Group 2)
- Micro-organisms that can cause severe human disease and are a serious hazard to employees, with possibility to spread to the community but with a usually effective treatment available (Hazard Group 3)
- These biological agents cause severe human disease and are a serious hazard to employees, they are also likely to spread to the community and have no effective treatment available (Hazard Group 4)
The Approved List of biological agents is a document by the HSE that is amended regularly to include micro-organisms in HG2, HG3 and HG4. Just because a biological agent isn’t on the list does not mean it is automatically part of HG1. Make sure you identify the Hazard Groups through a risk assessment. Biological agents also include genetically modified micro-organisms, but only those that pose a threat to human health. Remember, CoSHH only considers threats to human beings, and not environmental risks.
There are three ways biological agents might affect you, directly or indirectly, in the workplace:
- Exposure resulting from work with biological agents (microbiology laboratory)
- Exposure not resulting from the work itself but incidental to it (farming, refuse collection, sewage treatment)
- Exposure not resulting from the work that you do (catching flu from a work colleague)
CoSHH only covers the first two scenarios. The regulation assumes that the work with biological agents is deliberate, with incidental exposure to these micro-organisms. Apart from employees, CoSHH also considers any visitor, maintenance worker, engineer, patient, and cleaner entering these scenarios. The law treats students carrying out genetic modification as employees of the educational body where they study.
How to manage the exposure to biological agents while working in healthcare?
The most common and traditional way in healthcare to manage exposure to biological agents has been to follow the infection control policy. While the primary intention is to protect patients from the spread of infection, the control policy does also protect employees. It may include universal precautions, that help control exposure to blood and body fluids. It is important to apply these controls throughout all levels and areas of healthcare susceptible to exposure to biological agents. These include patient care (wards, surgeries, operating theatres) and service departments (sterile services, domestic services such as cleaning, laundry, portering).
These policies usually include protocols on hand washing, patient isolation, aseptic procedures, disinfection, and decontamination. While these are core procedures that help comply with Health & Safety but are not a result of workplace specific risk assessment directly. Risk assessment is very important to inform and train personnel on how to deal in specific situations relating to their workplace. That is why an individual assessment of the risks of infection and how to manage them is necessary. Each workplace should have their own health and safety policy and all employees and employers must understand it.
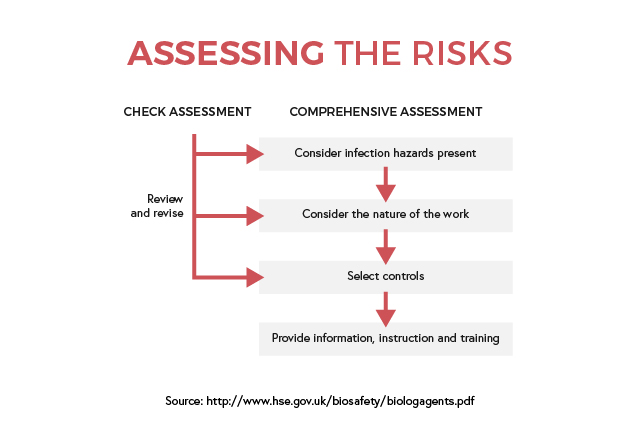
Depending on the size and complexity of the organisation, identifying the main infection hazards will be applicable for the whole building or specific rooms. The resulting findings will help in informing and training employees on how to manage exposure and risk of infections. Daily tasks and activities can be reviewed regularly, and any changes can be fed back into the assessment later.
What regulations & legislations cover working with biological agents in Healthcare?
Handling biological agents such as micro-organisms and infection risks in a healthcare setting can have many repercussions on public health if not done right. The level of risk is reflected in how many legislations and regulations frame the workplace to ensure proper handling and procedures. While the publications are not all issued by the same source, they can benefit in creating a safer workplace for all.
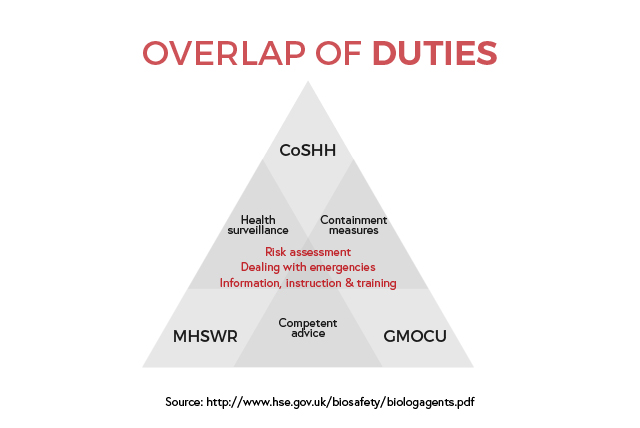
The primary legislation is the Health and Safety at Work etc Act (HSW – 1974), which encompasses all workplaces to generally ensure “so far as is reasonably practicable, the health, safety and welfare at work”. The Management of Health and Safety at Work Regulations (MHSWR – 1999) was introduced to reinforce HSW and places additional duties on employers, employees, and any contractors. The Control of Substances Hazardous to Health Regulations (CoSHH – 2002) which lists best practices to reduce exposure to hazardous substances. Three documents list more specific practices around the handling of hazardous substances:
- Reporting of Injuries, Diseases and Dangerous Occurrences Regulations (RIDDOR – 2013)
- Carriage of Dangerous Goods (Classification, Packaging and Labelling) Regulations (CDG – 1996)
- Genetically Modified Organisms (Contained Use) Regulations (GMOCU – 2014)
Other guidance documents provide recommendations and help support legislations and regulations. Three organisations currently offer guidance on handling biological agents in Healthcare.
Advisory Committee on Dangerous Pathogens (ACDP) Guidance:
- Biological Agents: Managing the Risks in Laboratories and Healthcare Premises
- Protection Against Blood-Borne Infection in the Workplace: HIV and Hepatitis
- Transmissible Spongiform Encephalopathy Agents: Safe Working and the Prevention of Infection
- Management and Control of Viral Haemorrhagic Fevers
Health and Safety Commission (HSC) / Health and Safety Executive (HSE) Guidance:
- Safe Disposal of Clinical Waste
- Safe Working and the Prevention of Infection in the Post Mortem Room
- Biological Agents: Managing the Risks in Laboratories and Healthcare Premises
Department of Health (DH) / National Health Service (NHS) Guidance:
- Guidance for Clinical Health Care Workers: Protection Against Infection with Blood-borne Viruses
- Hospital Infection Control
- The Prevention and Control of Tuberculosis in the United Kingdom
- Infection Control in the Build Environment
These documents offer guidance to healthcare and safety professionals, in public and private sectors. For any additional information, we highly recommend reading these documents. Wherever these regulations overlap, the general rule is to meet the more specific requirements.